
We report an experimental investigation on the formation of liquid core capsules having a thin hydrogel elastic membrane named liquid pearls. These fish-egg like structures are initially made of a millimetric liquid drop, aqueous or not, coated with an aqueous liquid film containing sodium alginate that gels once the double drop enters a calcium chloride bath. The creation of such pearls with micrometer thick membrane requires to suppress mixing until gelling takes place. Here, we show that superimposing a two dimensional surfactant precipitation at the interface confers a transient rigidity that can damp the shear induced instability at impact. Based on this, pearls containing almost any type of liquids can be created. This opens the possibility to use such structure as a new tool for screening microorganisms survival and growth in various three dimensional environment.
N. Bremond, E. Santanach-Carreras, L. Y. Chu, J. Bibette, 2010, Formation of liquid-core capsules having a thin hydrogel membrane: liquid pearls. Soft Matter, 6, 2484-2488.
N. Bremond, E. Santanach-Carreras, J. Bibette, 2011, Liquid pearls. Phys. Fluids, 23, 091108 (Gallery of fluid motion).
Capsules having a thin alginate hydrogel membrane and an aqueous core can be obtained by a process that involves a co-extrusion step in air followed by a sol-gel transition of the shell after immersion into a gelling bath. The possibility to encapsulate cells that further grow in these biocompatible compartments, and thus offer a versatile tool for cell culture, led us to investigate the physicochemical properties of the capsules. A cut-off pore size of the semi-permeable membrane is extrapolated from the release of polymers out of the capsule. When polymers can not diffuse through the membrane, the osmotic pressure mismatch between the core and the surrounding medium triggers an inflation of the capsule. The swelling may reach a steady state that allows to determine elastic features of the hydrogel shell. On the other hand, the capsule membrane may rupture and then contracts. From this stress-relaxation process, a critical deformation of the hydrogel shell above which plasticity occurs can be deduced. Finally, thanks to the physical nature of the hydrogel, the core content can be released by dissolving the membrane with the help of small electrolytes. The shell life is shown to vary inversely with the ionic strength of the solution.
L. Rolland,E. Santanach-Carreras, T. Delmas, J. Bibette, N. Bremond, 2014, Physicochemical properties of aqueous core hydrogel capsules. Soft Matter,10 (48), 9668-9674.
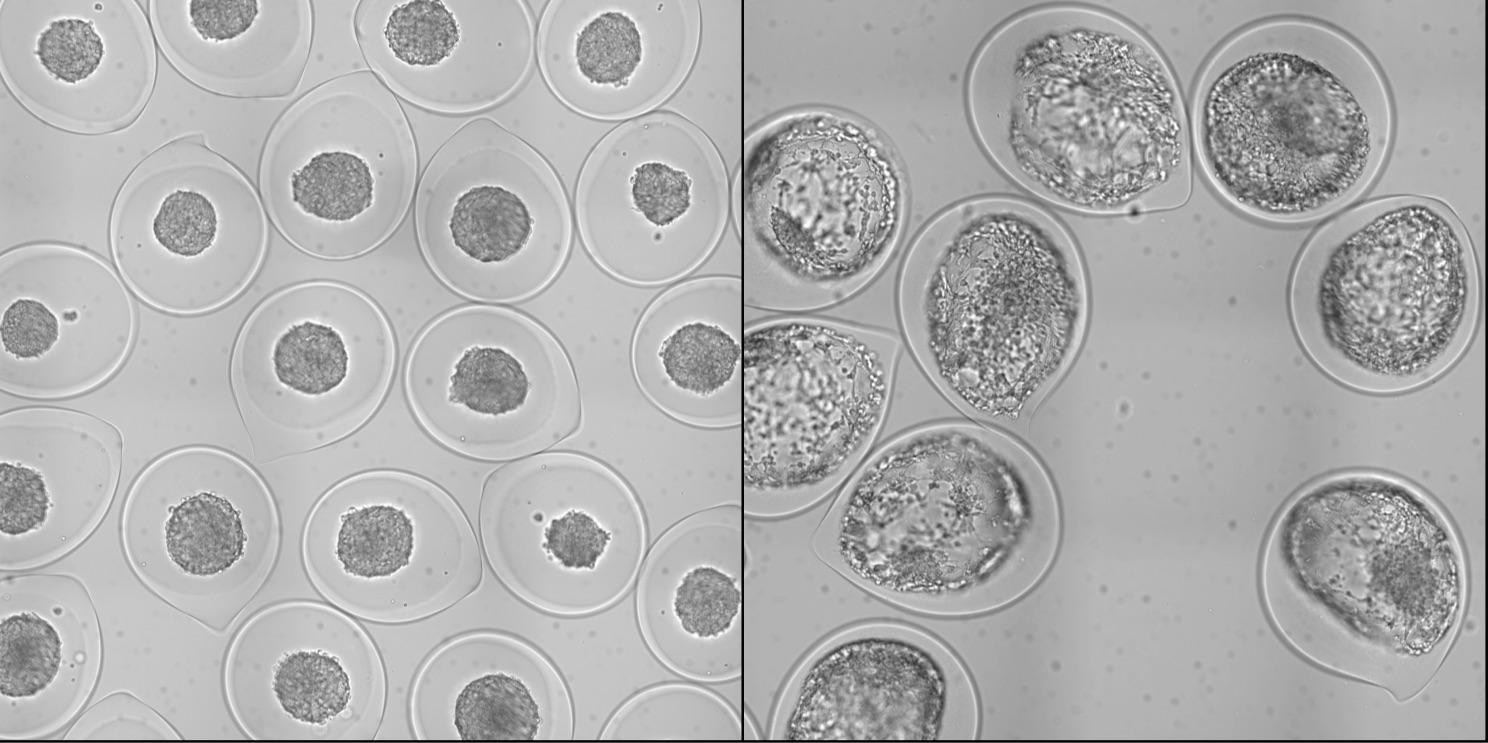
Deciphering the multifactorial determinants of tumor progression requires standardized high-throughput preparation of 3D in vitro cellular assays. We present a simple microfluidic method based on the encapsulation and growth of cells inside permeable, elastic, hollow microspheres. We show that this approach enables mass production of size-controlled multicellular spheroids. Due to their geometry and elasticity, these microcapsules can uniquely serve as quantitative mechanical sensors to measure the pressure exerted by the expanding spheroid. By monitoring the growth of individual encapsulated spheroids after confluence, we dissect the dynamics of pressure buildup toward a steady-state value, consistent with the concept of homeostatic pressure. In turn, these confining conditions are observed to increase the cellular density and affect the cellular organization of the spheroid. Postconfluent spheroids exhibit a necrotic core cemented by a blend of extracellular material and surrounded by a rim of proliferating hypermotile cells. By performing invasion assays in a collagen matrix, we report that peripheral cells readily escape preconfined spheroids and cell–cell cohesivity is maintained for freely growing spheroids, suggesting that mechanical cues from the surrounding microenvironment may trigger cell invasion from a growing tumor. Overall, our technology offers a unique avenue to produce in vitro cell-based assays useful for developing new anticancer therapies and to investigate the interplay between mechanics and growth in tumor evolution.
K. Alessandri et al., 2013, Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Nat. Acad. Sci. USA, 111 (22), 8055-8060.
Liquid core capsules having a hydrogel membrane are becoming a versatile tool for three-dimensional cul- ture of micro-organisms and mammalian cells. Making sub-millimeter capsules at a high rate, via the breakup of a compound jet in air, opens the way to high-throughput screening applications. However, control of the capsule size monodispersity, especially required for quantitative bioassays, was still lacking. Here, we report how the understanding of the underlying hydrodynamic instabilities that occur during the process can lead to calibrated core–shell bioreactors. The requirements are: i) damping the shear layer in- stability that develops inside the injector arising from the co-annular flow configuration of liquid phases having contrasting viscoelastic properties; ii) controlling the capillary instability of the compound jet by superposing a harmonic perturbation onto the shell flow; iii) avoiding coalescence of drops during jet frag- mentation as well as during drop flight towards the gelling bath; iv) ensuring proper engulfment of the compound drops into the gelling bath for building a closed hydrogel shell. We end up with the creation of numerous identical compartments in which cells are able to form multicellular aggregates, namely spher- oids. In addition, we implement an intermediate composite hydrogel layer, composed of alginate and colla- gen, allowing cell adhesion and thus the formation of epithelia or monolayers of cells.
H. Doméjean, M. de la Motte Saint Pierre, A. Funfak, N. Atrux-Tallau, K. Alesandri, P. Nassoy J. Bibette and N. Bremond, 2017, Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture Lab Chip, 17, 110–119.
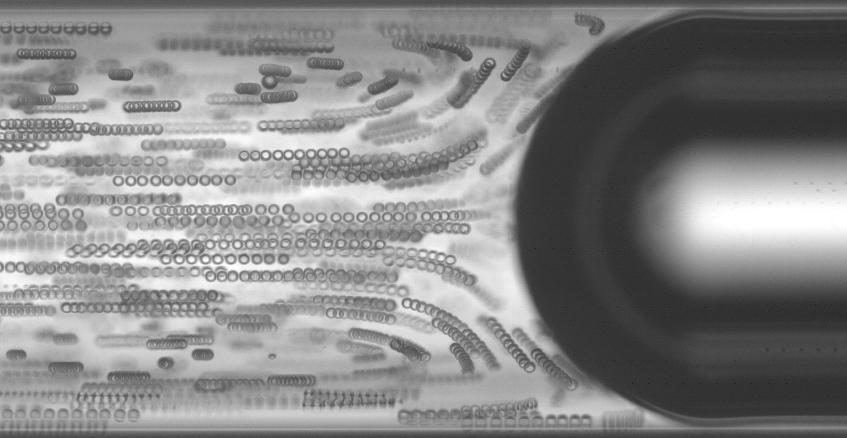
Convective dispersion of solutes is inherent to flow in channels because of the nonuni- formity of the velocity profile. When diffusion is negligible, for large particles for example, the trajectory of particles can be solely described by a kinematic approach. Here, we investigate such a phenomenon for micrometer-size beads flowing in a circular pipe. We show that the presence of large bubbles, namely in the case of a segmented flow, either prevents the convective dispersion or leads to the accumulation of particles at the rear of the bubble moving in front. The destabilization of the initially homogeneous suspension occurs when liquid inertia comes into play. Indeed, for moderate Reynolds number of the particles, particles move away from the wall, thus exploring different flow lines that finally impact the axial dispersion features. Moreover, since the bubbles impose an axial boundary condition of the mean velocity, a net flux of particles directed along the flow direction is built up above a critical particle Reynolds number. This work is motivated by the understanding of the flow behavior of biological samples, and especially in the context of cell encapsulation.
W. Bouhlel, S.D. Naghib, J. Bibette, N. Bremond, 2019, Convective dispersion of particles in a segmented flow. Phys. Rev. Fluids, 111 (4), 104303.

Some bacteria can act as catalysts to oxidize (or reduce) organic or inorganic matter with the potential of generating electrical current. Despite their high value for sustainable energy, organic compound production and bioremediation, a tool to probe the natural biodiversity and to select most efficient microbes is still lacking. Compartmentalized cell culture is an ideal strategy for achieving such a goal but the appropriate compartment allowing cell growth and electron exchange must be tailored. Here, we develop a conductive composite hydrogel made of a double network of alginate and carbon nanotubes. Homogeneous mixing of carbon nanotubes within the polyelectrolyte is obtained by a surfactant assisted dispersion followed by a desorption step for triggering electrical conductivity. Dripping the mixture in a gelling bath through simple extrusion or a double one allows the formation of either plain hydrogel beads or liquid core hydrogel capsules. The process is shown to be compatible with the bacterial culture (Geobacter sulfurreducens). Bacteria can indeed colonize the outer wall of plain beads or the inner wall of the conductive capsules’ shell that function as an anode from which electrons produced by the cells are collected.
L. Mottet, D. Le Cornec, J.-M. Noël, F. Kanoufi, B. Delord, P. Poulin, J. Bibette and N. Bremond, 2018, A conductive hydrogel based on alginate and carbon nanotubes for probing microbial electroactivity. Soft Matter, 14, 1434.
J.-M. Noël, L. Mottet, N. Bremond, P. Poulin, C. Combellas, J. Bibette, F. Kanoufi, 2015, Multiscale electrochemistry of hydrogels embedding conductive nanotubes. Chem. Sci., 6, 3900.
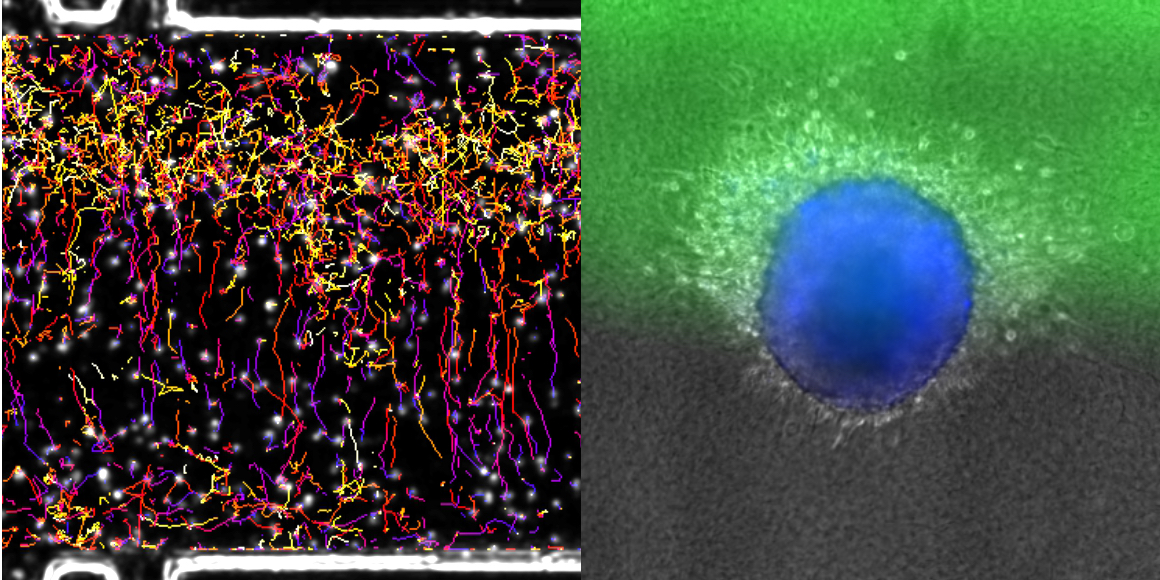
In many cell types, migration can be oriented towards a chemical stimulus. In mammals, for example, em- bryonic cells migrate to follow developmental cues, immune cells migrate toward sites of inflammation, and cancer cells migrate away from the primary tumour and toward blood vessels during metastasis. Understanding how cells migrate in 3D environments in response to chemical cues is thus crucial to understanding directed migration in normal and disease states. To date, chemotaxis in mammalian cells has been primarily studied using 2D migration models. However, it is becoming increasingly clear that the mechanisms by which cells migrate in 2D and 3D environments dramatically differ, and cells in their native environments are confronted with a complex chemical milieu. To address these issues, we developed a microfluidic device to monitor the behaviour of cells embedded in a 3D collagen matrix in the presence of complex concentration fields of chemoattractants. This tuneable microsystem enables the generation of (1) homogeneous, stationary gradients set by a purely diffusive mechanism, or (2) spatially evolving, stationary gradients, set by a convection–diffusion mechanism. The device allows for stable gradients over several days and is large enough to study the behaviour of large cell aggregates. We observe that primary mature dendritic cells respond uniformly to homogeneous diffusion gradients, while cell behaviour is highly position-dependent in spatially variable convection–diffusion gradients. In addition, we demonstrate a directed response of cancer cells migrating away from tumour-like aggregates in the presence of soluble chemokine gradients. Together, this microfluidic device is a powerful system to observe the response of different cells and aggregates to tuneable chemical gradients.
K. Aizel, A. G. Clark, A. Simon, S. Geraldo, A. Funfak, P. Vargas, J. Bibette, D. Matic Vignjevic and N. Bremond, 2017, A tuneable microfluidic system for long duration chemotaxis experiments in a 3D collagen matrix. Lab Chip, 109, 7181.
Microorganisms are widely used to generate valuable products and their efficiency is a major industrial focus. Bioreactors are typically composed of billions of cells and available measurements only reflect the overall performance of the population. However, cells do not equally contribute and process optimization would therefore benefit from monitoring this intra-population diversity. This has so far remained difficult because of the inability to probe concentration changes at the single cell level. Here, we unlock this limitation by taking advantage of the osmotically driven water flux between a droplet containing a living cell towards surrounding empty droplets, within a concentrated inverse emulsion. With proper formulation, excreted products are far more soluble within the continuous hydrophobic phase compared to initial nutrients (carbohydrates and salts). Fast diffusion of products induces an osmotic missmatch, which further relaxes due to slower diffusion of water through hydrophobic interfaces. By measuring droplet volume variations, we can deduce the metabolic activity down to isolated single cells. As a proof of concept, we present the first direct measurement of the maintenance energy of individual yeast cells. This method does not require any added probes and can in principle apply to any osmotically sensitive bioactivity, opening new routes for screening and sorting large libraries of microorganisms and biomolecules.
L. Boitard , D. Cottinet, C. Kleinschmitt, N. Bremond, J. Baudry, G. Yvert and J. Bibette, 2012, Monitoring Single Cell Bioenergetics via the Coarsening of Emulsion Droplets. Proc. Natl. Acad. Sci. USA, 109, 7181.

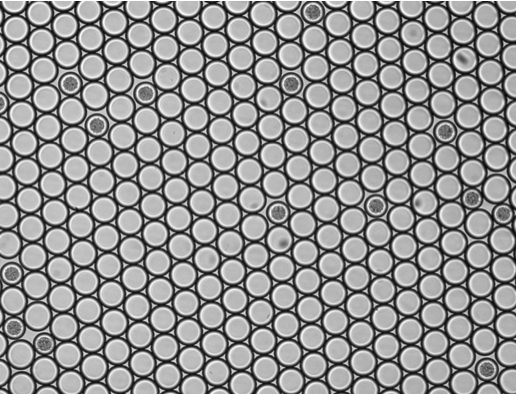
We present a novel millifluidic droplet analyser (MDA) for precisely monitoring the dynamics of microbial populations over multiple generations in numerous (≥10^3) aqueous emulsion droplets (~100 nL). As a first application, we measure the growth rate of a bacterial strain and determine the minimal inhibitory concentration (MIC) for the antibiotic cefotaxime by incubating bacteria in a fine gradient of antibiotic concentrations. The detection of cell activity is based on the automated detection of an epifluorescent signal that allows the monitoring of microbial populations up to a size of ~106 cells. We believe that this device is helpful for the study of population dynamic consequences of microbe-environment interactions and of individual cell differences. Moreover, the fluidic machine may improve clinical tests, as it simplifies, automates and miniaturizes the screening of numerous microbial populations that grow and evolve in compartments with a finely tuned composition.
L. Baraban, F. Bertholle, M. L.M. Salverda, N. Bremond, P. Panizza, J. Baudry, J. A. G.M. de Visser and J. Bibette, 2011, Millifluidic droplet analyser for microbiology. Lab Chip, 11, 4057.
S. P. Damodaran, S. Eberhard, L. Boitard, J. Garnica Rodriguez, Y. Wang, N. Bremond, J. Baudry, J. Bibette, F.-A. Wollman, 2015, A millifluidic study of cell-to-cell heterogeneity in growth-rate and cell-division capability in populations of isogenic cells of Chlamydomonas reinhardtii. PloS one, 10 (3), e0118987.
L. Boitard, D. Cottinet, N. Bremond, J. Baudry, J. Bibette, 2015, Growing microbes in millifluidic droplets. Eng. Life Sci., 10 (3), e0118987.
D. Cottinet, F. Condamine, N. Bremond, A. D. Griffiths, P. B. Rainey, J A. G. M. de Visser, J. Baudry, J. Bibette, 2016, Lineage Tracking for Probing Heritable Phenotypes at Single-Cell Resolution. PloS one, 0152395.