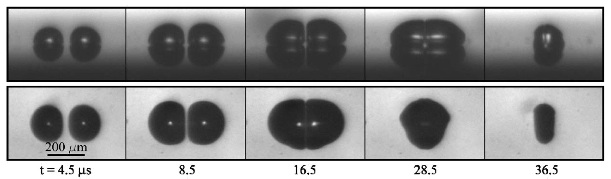
We report experimental and numerical investigations on the dynamics of the cavitation of bubbles on a solid surface and the interaction between them with the help of controlled cavitation nuclei: hemispherical bubbles are nucleated from hydrophobic microcavities that act as gas traps when the substrate is immersed in water. The expansion of these nuclei is triggered by an impulsive lowering of the liquid pressure. The patterning of the substrate allows us to control the number of bubbles and the distance between them. Each hemispherical bubble experiences the effect of its mirror image. Correspondingly, an isolated hemispherical bubble together with its mirror image behaves like a free spherical bubble, i.e., its dynamics is well described by the Rayleigh-Plesset equation. We employ the setup to study the dynamics of two and more bubbles in a row at controlled and fixed distances from each other. For weak interaction, namely when the maximum size of the bubbles is smaller than the bubble distance, the dynamics of the system is well captured by an extended Rayleigh-Plesset equation, where mutual pressure coupling through sound emission is included. Bubble pairs last longer than an isolated bubble as neighboring bubbles modify the surrounding pressure and screen each other. For strong interaction, obtained by increasing the tensile stress or decreasing the bubble distance, the bubbles eventually flatten and form a liquid film between each other which can rupture, leading to coalescence. The film thinning is inertia dominated. A potential flow boundary integral simulation captures the overall shape evolution of the bubbles, including the formation of jets horizontal to the wall. These horizontal jets are caused by symmetry breaking due to the neighboring bubbles.
N. Bremond, M. Arora, S. M. Dammer, D. Lohse, 2006, Interaction of cavitation bubbles on a wall. Phys. Fluids, 18, 121505.
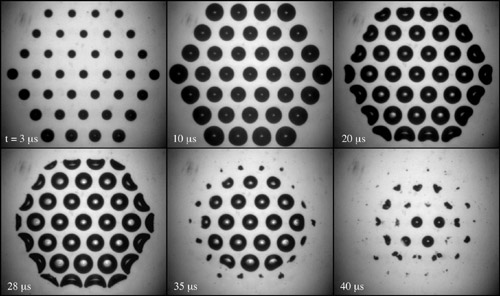
Heterogeneous bubble nucleation at surfaces has been notorious because of its irreproducibility. Here controlled multibubble surface cavitation is achieved by using a hydrophobic surface patterned with microcavities. The expansion of the nuclei in the microcavities is triggered by a fast lowering of the liquid pressure. The procedure allows us to control and fix the bubble distance within the bubble cluster. We observe a perfect quantitative reproducibility of the cavitation events where the inner bubbles in the two- dimensional cluster are shielded by the outer ones, reflected by their later expansion and their delayed collapse. Apart from the final bubble collapse phase (when jetting flows directed towards the cluster’s center develop), the bubble dynamics can be quantitatively described by an extended Rayleigh-Plesset equation, taking pressure modification through the surrounding bubbles into account.
N. Bremond, M. Arora, C. D. Ohl, D. Lohse, 2006, Controlled multi-bubble surface cavitation. Phys. Rev. Lett., 96, 224501.
N. Bremond, M. Arora, C. D. Ohl, D. Lohse, 2005, Cavitation on surfaces. J. Phys.: Condens. Matter, 49, S3603-S3608.
N. Bremond, M. Arora, C. D. Ohl, D. Lohse, 2005, Cavitation on patterned surfaces. Phys. Fluids, 17, 091111, (Gallery of fluid motion).
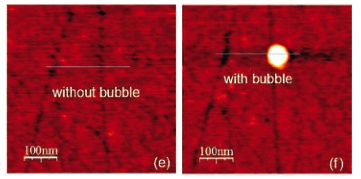
The aim of this paper is to quantitatively characterize the appearance, stability, density, and shape of surface nanobubbles on hydrophobic surfaces under varying conditions such as temperature and temperature variation, gas type and concentration, surfactants, and surface treatment. The method we adopt is atomic force microscopy (AFM) operated in the tapping mode. In particular, we show (i) that nanobubbles can slide along grooves under the influence of the AFM tip, (ii) that nanobubbles can spontaneously form by substrate heating, allowing for a comparison of the surface topology with and without the nanobubble, (iii) that a water temperature increase leads to a drastic increase in the nanobubble density, (iv) that pressurizing the water with CO2 also leads to a larger nanobubble density, but typically to smaller nanobubbles, (v) that alcohol-cleaning of the surface is crucial for the formation of surface nanobubbles, (vi) that adding 2-butanol as surfactant leads to considerably smaller surface nanobubbles, and (vii) that flushing water over alcohol-covered surfaces strongly enhances the formation of surface nanobubbles.
S. Yang, S.M. Dammer, N. Bremond, H. J. W. Zandvliet, E. S. Kooij, D. Lohse, 2007, Characterization of nanobubbles on hydrophobic surfaces in water. Langmuir, 23, p. 7072-7077.