Emulsions have been studied for a long time because of the richness of their related fundamental physicochemical phenomena and owing to their wide industrial applications. The development of microfluidics offers new opportunities to investigate emulsion features and behaviours. This review relates the use of microfluidic tools for probing the interfacial properties of emulsion droplets and the two main mechanisms of destabilisation, namely the coalescence of adjacent drops and the molecular transfer between neighbouring drops.
N. Bremond, J. Bibette, 2012, Exploring emulsion science with microfluidics. Soft Matter, 8, 10549-10559.
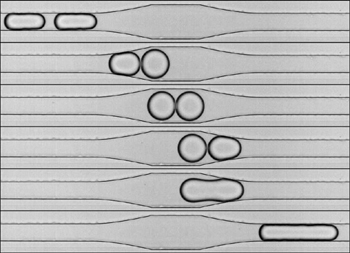
The destabilization process of an emulsion under flow is investigated in a microfluidic device. The experimental approach
enables us to generate a periodic train of droplet pairs, and thus to isolate and analyze the basic step of the destabilization,
namely the coalescence of two droplets which collide. We demonstrate a counter intuitive phenomenon: coalescence occurs during the
separation phase and not during the impact. Separation induces the formation of two facing nipples in the contact area that hastens
the connexion of the interfaces prior to fusion. Moreover, droplet pairs initially stabilized by surfactants can be destabilized by
forcing the separation.
Finally, we note that the fusion mechanism is responsible of a cascade of coalescence events in a compact system of droplets where
the separation is driven by surface tension.
N. Bremond, A. R. Thiam, J. Bibette, 2008, Decompressing emulsion droplets favors coalescence. Phys. Rev. Lett., 100, 024501.

see also the story in Physical Review Focus
Recent microfluidic experiments by Bremond et al. (2008), along with simulations by Yoon et al. (2007) as well as near contact experiments and simulations by Manica et al. (2008), have demonstrated that two droplets can coalesce as they are separating rather than upon their collision. We analyze the experimental microfluidic flow configuration for the approach to contact with a two-dimensional model: we apply a lubrication analysis followed by the method of domain perturbation to determine the droplet deformation as a function of time. We find the approximate shape for the deformed droplet at the time of contact. In particular, for droplets of radius, R, moving apart according to h_0(t)=h_0(0)+\alpha t^2, where 2h_0(t) is the separation distance, we define a non-dimensional parameter, A=\frac{4C\mu R^2\alpha^{1/2}}{\pi \gamma [h_0(0)]^{3/2}}, where \mu is the viscosity of the continuous phase, \gamma is the interfacial tension, and C depends on the viscosity ratio between the droplets and the continuous phase. Our model suggests that there exists a critical value, A_{crit}=\frac{16}{3^{3/2}} ~ 3.0792, below which separation is unlikely to facilitate the coalescence of the droplets. The predictions are in good agreement with available experimental data.
A. Lai, N. Bremond, H. A. Stone, 2009, Separation-driven coalescence of droplets: An analytical criterion for the approach to contact. J. Fluid Mech., 632, p. 97-107.
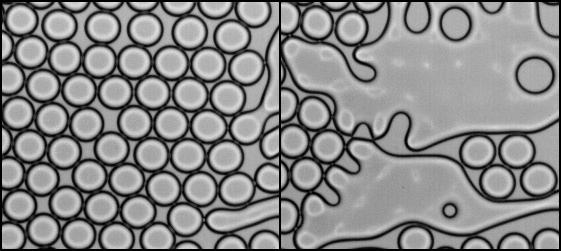
The phase inversion that undergoes an emulsion while being sheared is a sudden phenomenon that is still puzzling. In this Letter, we report an experimental investigation on propagative coalescence by using a microfluidic device where a calibrated two-dimensional emulsion is created and destabilized. The velocity of propagation as well as the probability of the coalescence are reported as a function of the size and the spatial distribution of the drops. We then discuss the efficiency of this novel scenario of phase inversion and suggest that inversion can be favored by the existence of a drop size distribution.
N. Bremond, H. Doméjean, J. Bibette, 2011, Propagation of drop coalescence in a two-dimensional emulsion: A route towards phase inversion. Phys. Rev. Lett., 106, 214502.

By using microfluidic chips, we investigate the stability regarding coalescence of droplet pairs under electric field as a function of drop separation and ac field intensity. Three different regimes are found: stable, coalescence and partial merging. From this, we identify the two breaking scenarii of a one dimensional train of droplets: in one case the coalescence front propagates, in the other case, which for pairs corresponds to the partial merging regime, the coalescence front can become heterogeneous. From these findings, we can propose a destruction mechanism for a macroscopic emulsion, which includes the packing condition for which total and immediate destruction is effective.
A. R. Thiam, N. Bremond, J. Bibette, 2009, Breaking of an emulsion under an ac electric field. Phys. Rev. Lett., 102, 188304.
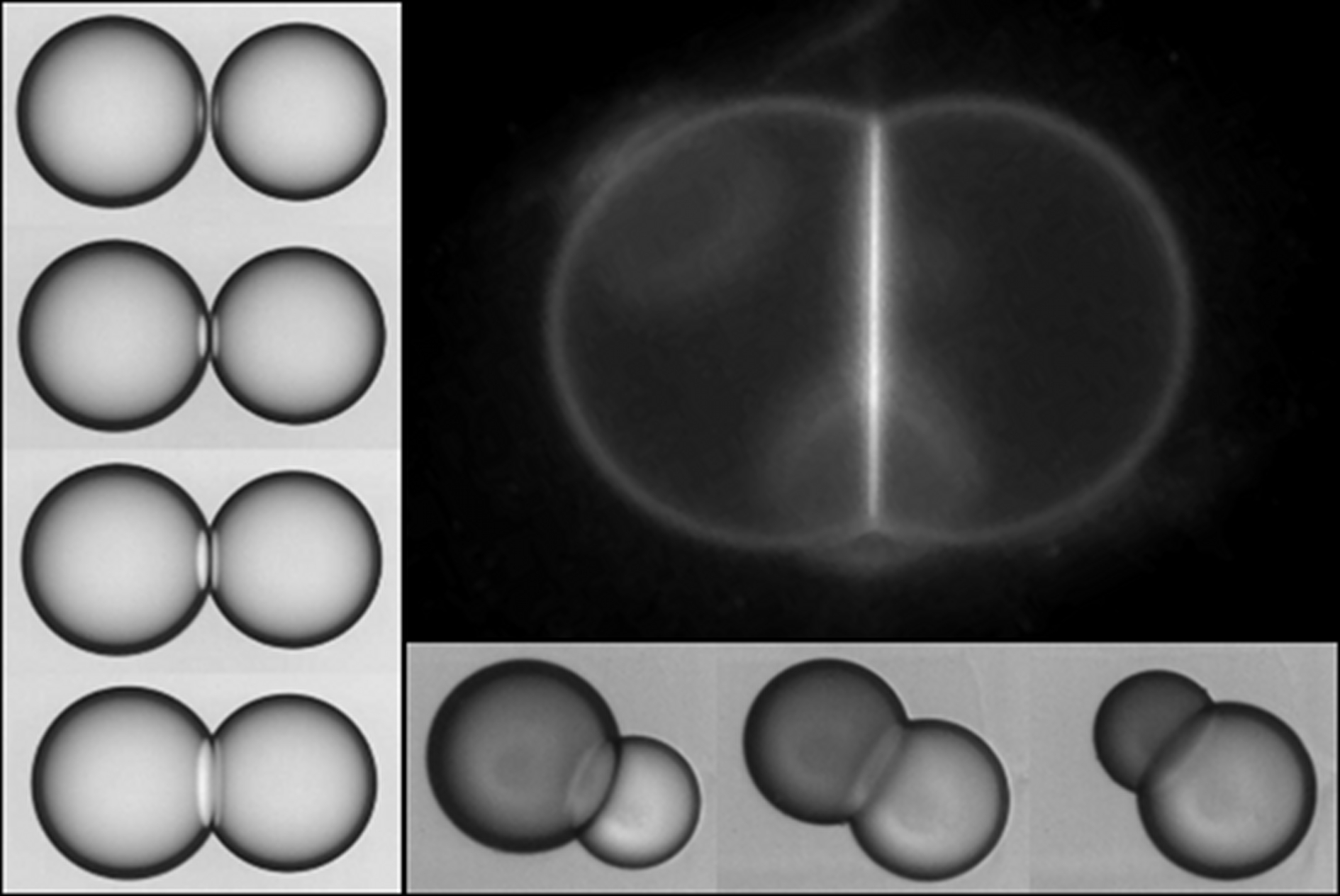
Water-in-oil emulsion drops are formed and stabilized with phospholipids which can adhere and form a bilayer. By using microfluidic technologies, we probe the stability and properties of such membranes likewise encountered in foams or vesicles. First, adhesive drop pairs are then trapped and submitted to an ac electric field. We observe three distinct states as a function of the adhesion energy and the electric field intensity. The pair can be either stable, though slightly deformed, or unzip and separate, or coalesce. The frontiers between the different states directly reflect vesicle detachment forces and electroporation theories. The experimental approach that we propose for probing liquid interface wetting between monolayers allows us to finely tuned the tension in the bilayer and gives access to bilayer unzipping. We then establish the stability diagram of adhering drop pairs as a function of the continuous phase composition. We found two regimes of destabilization of the bilayer. The first one concerns a competition between the dynamics of adhesion and the transport of surfactants towards the interfaces that leads to a dilute surfactant coverage. The second one corresponds to a dense surface coverage where the lifetime distribution of the bilayer exponentially decreases as a signature of a nucleation process. In the stable regime, we observe the propagation of adhesion among a concentrated collection of drops. This is another remarkable illustration of the suction consequence when two close deformable objects are pulled apart. Moreover, the present experimental strategy offers a novel way to study the phase diagrams of bilayers from a single phospholipid to a mixture of phospholipids. Indeed, we detect phase transitions at a liquid-liquid interface that are ruled by the amount of bad solvent. Finally, we probe the transport of water molecules through the bilayer and show that its permeability is linked to the adhesion energy that reflects its fluidity.
A. R. Thiam, N. Bremond, J. Bibette, 2011, Adhesive emulsion bilayers under an electric field : from unzipping to fusion. Phys. Rev. Lett., 107, 068301.
A. R. Thiam, N. Bremond, J. Bibette, 2012, From stability to permeability of adhesive emulsion bilayers. Langmuir, 28 6291.
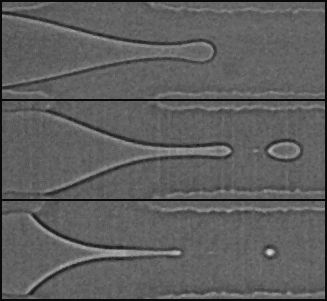
The capacity of microfluidic technology to fabricate monodisperse emulsion droplets is well established. Parallelization of droplet production is a prerequisite for using such an approach for making high quality materials for either fundamental or industrial applications where product quantity matters. Here, we investigate the emulsification efficiency of parallelized drop generators based on a flow focusing geometry when incorporating the role of partial wetting in order to make emulsion droplets with a diameter below 10 microns. Confinement intrinsically encountered in microsystems intensifies the role played by interfaces between liquids and solids. We thus take advantage of partial wetting to enhance the maximum confinement accessible due to liquid flow focusing. We compare the performances brought by partial wetting to more established routes such as step emulsification. We show that the step configuration and the partial wetting regime are both well suited for being parallelized and thus open the way to the production of fine and calibrated emulsions for further applications. Finally, this new route of emulsification that exploits partial wetting between the fluids and the channel walls opens possibilities to the formation of substantially smaller droplets, as required in many fields of application.
C. Cohen et al., 2014, Parallelised production of fine and calibrated emulsions by coupling flow focusing technique and partial wetting phenomenon. Microfluid. Nanofluid., 17, 959–966.
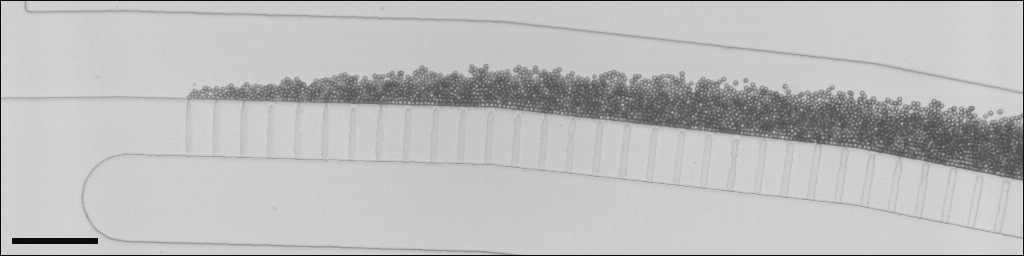
Microfluidics has proven to be an efficient tool for making fine and calibrated emulsion droplets. The parallelization of drop makers is required for producing large amounts. Here, we investigate the generation of emulsion drops along a series of shallow microchannels emerging in a deep one, in other words the step-emulsification process. The dynamics of a single drop maker is first characterized as a function of interfacial tension and viscosities of both phases. The characteristic time scale of drop formation, namely, the necking time that finally sets drop size, is shown to be principally governed by the outer phase viscosity to interfacial tension ratio with a minor correction linked to the viscosity ratio. The step emulsification process experi- ences a transition of fragmentation regime where both the necking time and drop size suddenly raise. This transition, that corresponds to a critical period of drop formation and thus defines a maximum production rate of small droplets, is observed to be ruled by the viscosity ratio of the two phases. When drops are produced along an array of microchannels with a cross flow of the continuous phase, a configuration comparable to a one-dimensional membrane having rectangular pores, a drop boundary layer develops along the drop generators. In the small drop regime, the local dynamics of drop formation is shown to be independent on the emulsion cross flow. Moreover, we note that the development of the drop boundary layer is even beneficial to ho- mogenize drop size along the production line. On the other hand, in the large drop regime, drop collision can trigger fragmentation and thus lead to size polydispersity.
N. Mittal, C. Cohen, J. Bibette, N. Bremond, 2014, Dynamics of step-emulsification: From a single to a collection of emulsion droplet generators. Phys. Fluids,26, 082109
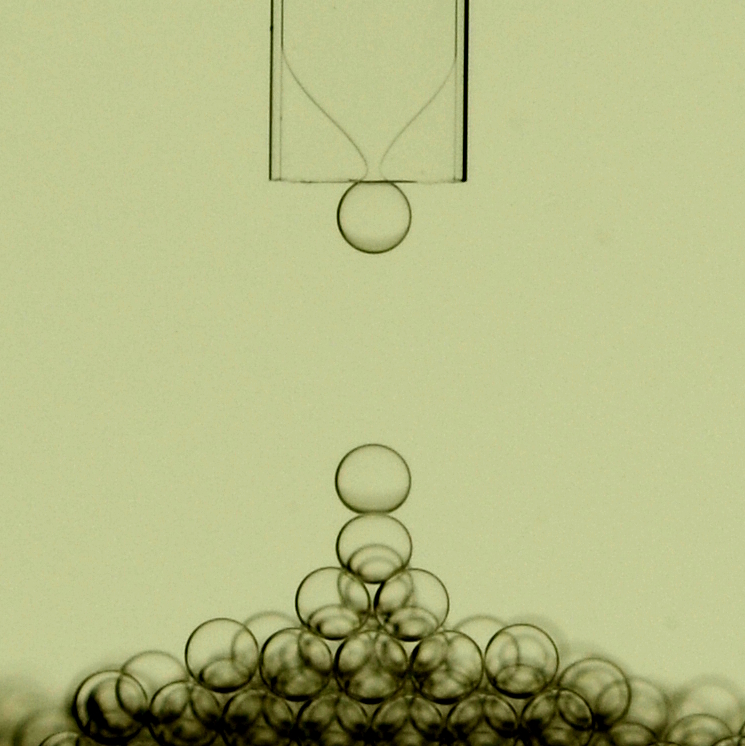
The flow of two immiscible liquids or fluids in bounded systems where confinement geometry varies can lead to drop or bubble formation. This phenomenon has been reported in the context of oil recovery and named snap-off, or exploited for making emulsions, and then foams, by using microfluidic systems, namely, microchannel emulsification or step emulsification. We report a comprehensive experimental investigation of such an emulsification process occurring at the end of a glass rectangular tube filled with oil and immersed in a water bath. This allows us to clearly visualize the breakup event of the dispersed phase liquid finger at the capillary’s end. Below a critical flow rate, the drop size varies slowly with the flow rate and it is linked to the pinching time of the dispersed phase. A semiempirical law that gives the resulting drop size as a function of fluid and geometrical properties is proposed. However, this feature is altered for an aspect ratio of the rectangular tube below 2.5 where the forming drop hinders the counterflow of the continuous phase leading to larger drops. Then, above a critical flow rate, or capillary number that weakly depends on the viscosity ratio of the two liquids, the neck adopts a quasistatic shape well accounted for by a model based on a Hele-Shaw flow. In that case, drop formation is driven by gravity and a transition from a dripping regime to a jetting regime is observed at higher flow rates. Monodisperse foam can also be formed by injecting air. While the overall dynamics of bubble formation shares similarities with an incompressible fluid, the bubble size and the critical capillary number do not follow the same scaling laws.
E. Crestel, L. Derzsi, H. Bartolomei, J. Bibette, N. Bremond, 2019, Emulsification with rectangular tubes. Phys. Rev. Fluids,4, 073602

The fabrication of alginate hydrogel microparticles with embedded liposomes and magnetic nanoparticles for radiofrequency controlled release of encapsulated chemical cargo was considered. An extractive gelation process was implemented in a microfluidic device, which enabled the production of uniform composite microparticles of dimensions comparable to those of blood cells (between 5 and 10 microns). The critical parameters that control the extractive gelation process were systematically explored and feasible values that provide microgel particles of a defined size and morphology were identified. First, the initial water-in-oil droplet is formed in a flow-focusing junction whose size is controlled by the flow-rate of the oil phase. Then, the train of droplets is sandwiched between two streams of oil containing calcium ions. In that way, a flux of water molecules from the droplets towards the continuous phase as well as a transport of calcium ions towards the disperse phase are initiated. The final microparticle properties were thus found to be sensitive to three elementary sub-processes: (i) the initial droplet size; (ii) the extraction of water into the oil phase, which was controlled by the volume of the oil phase and its initial moisture content; and (iii) the kinetics of ionic cross-linking of the alginate matrix, which was controlled by the varying calcium concentration. The size and morphology of the final composite microgels were fully characterized.
A. Pittermannova et al., 2016, Microfluidic fabrication of composite hydrogel microparticles in the size range of blood cells. RSC Adv., 6, 103532.
A. Pittermannova et al., 2020, Functionalized hydrogel microparticles prepared by microfluidics and their interaction with tumour marker carbonic anhydrase IX. Soft Matter, 16, 8702-8709.
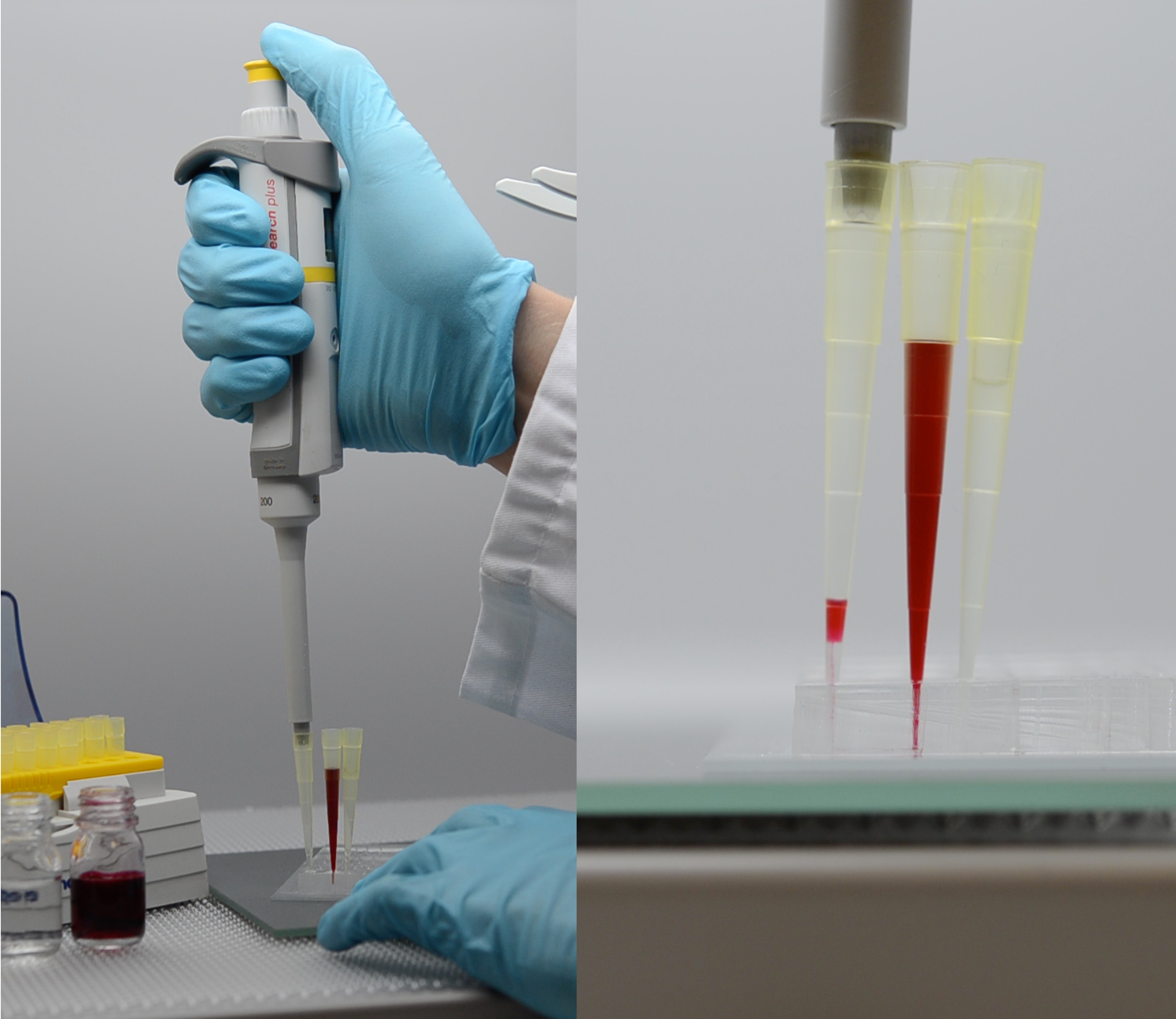
Droplet-based microfluidics, using water-in-oil emulsion droplets as micro-reactors, is becoming a widespread method for performing assays and especially in the cell biol- ogy field. Making a simple and highly portable system for creating emulsion droplets would help to continue the popularization of such a technique. Also, the ability to emulsify all the samples would strengthen this compartimenlization technique to han- dle samples with limited volume. Here, we propose a strategy of droplet formation that combines a classical flow-focusing microfluidic chip, which could be commer- cially available, with a standard laboratory adjustable micropipette. The micropipette is used as a negative pressure generator for controlling liquid flows. In that way, emul- sification does neither require any electrical power supply nor a cumbersome device and functions with small liquid volumes. Droplet formation can be easily and safely performed in places with limited space, opening a wide range of applications espe- cially in biological laboratory environments with higher level of safety regulations, i.e., BSL-3/4. Fortunately, the present methodology that involves small fluid vol- umes, and thus possible time dependent flow conditions, allows to minimize dead volume while keeping drops’ size homogeneous. A physical characterization of droplet production and a model that describes the emulsion features, in terms of drop size and size distribution, are proposed for rationalizing the performances of the micropipette-powered emulsification process.
K. Langer, N. Bremond, L. Boitard, J. Baudry, and J. Bibette, 2018, Micropipette-powered droplet based microfluidics. Biomicrofluidics, 12, 044106.
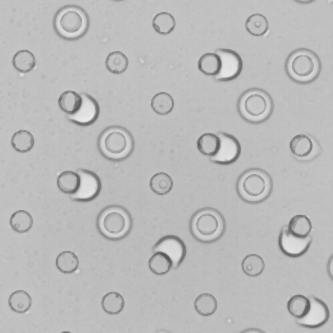
The capacity of microfluidic technology to fabricate monodisperse emulsion droplets is well established. Parallelization of droplet production is a prerequisite for using such an approach for making high quality materials for either fundamental or industrial applications where product quantity matters. Here, we investigate the emulsification efficiency of parallelized drop generators based on a flow focusing geometry when incorporating the role of partial wetting in order to make emulsion droplets with a diameter below 10 microns. Confinement intrinsically encountered in microsystems intensifies the role played by interfaces between liquids and solids. We thus take advantage of partial wetting to enhance the maximum confinement accessible due to liquid flow focusing. We compare the performances brought by partial wetting to more established routes such as step emulsification. We show that the step configuration and the partial wetting regime are both well suited for being parallelized and thus open the way to the production of fine and calibrated emulsions for further applications. Finally, this new route of emulsification that exploits partial wetting between the fluids and the channel walls opens possibilities to the formation of substantially smaller droplets, as required in many fields of application.
E. Crestel, A Kvasničková, E Santanach-Carreras, J. Bibette, N. Bremond, 2020, Motion of oil in water induced by osmosis in a confined system. Phys. Rev. Fluids, 5, 104003.